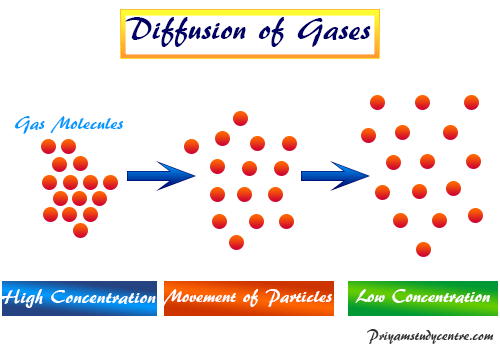
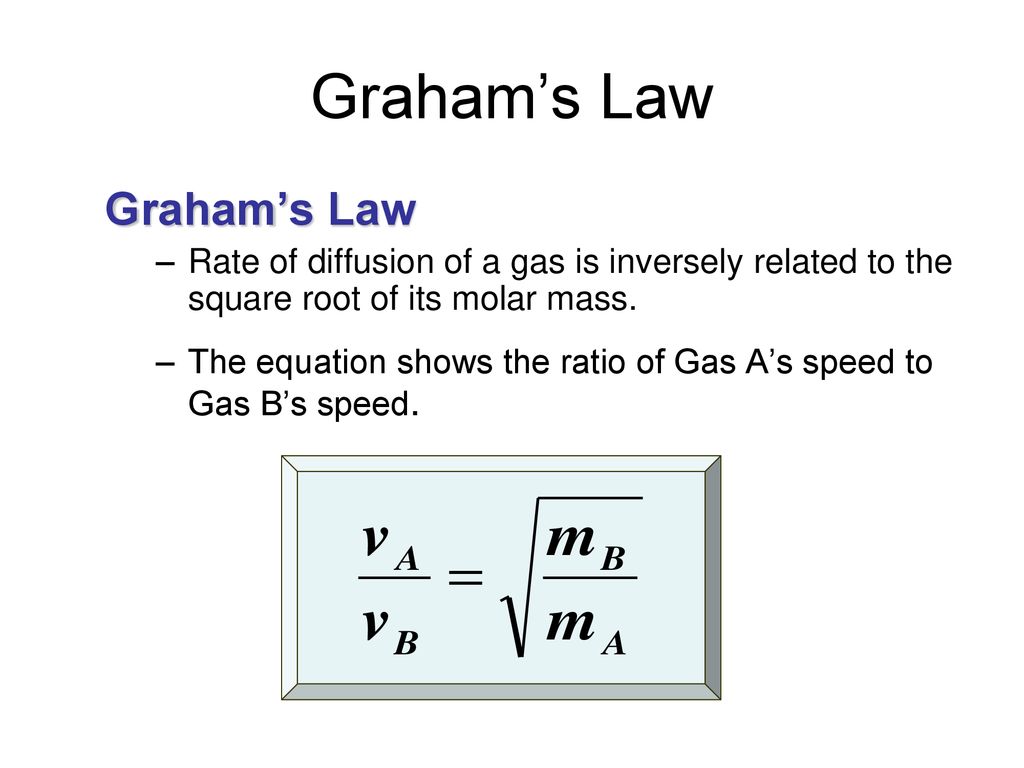
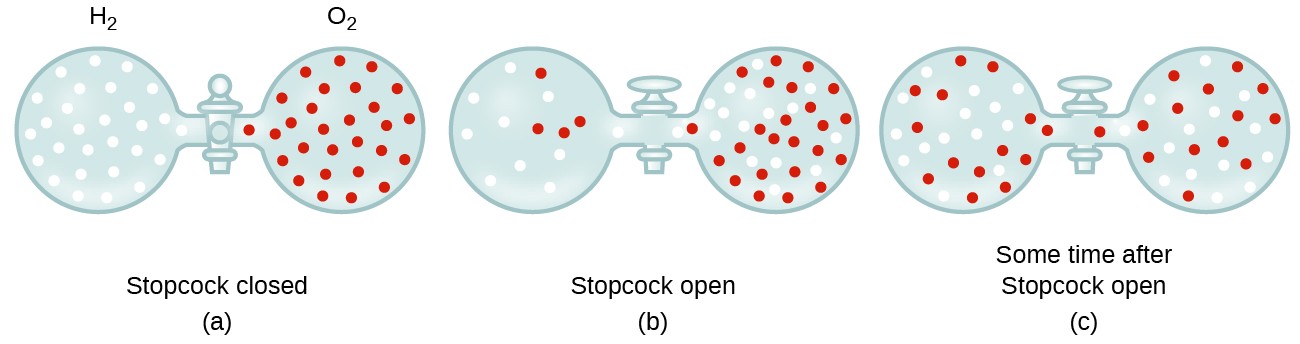
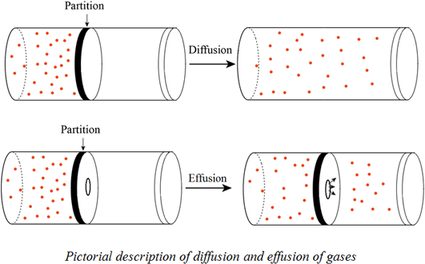
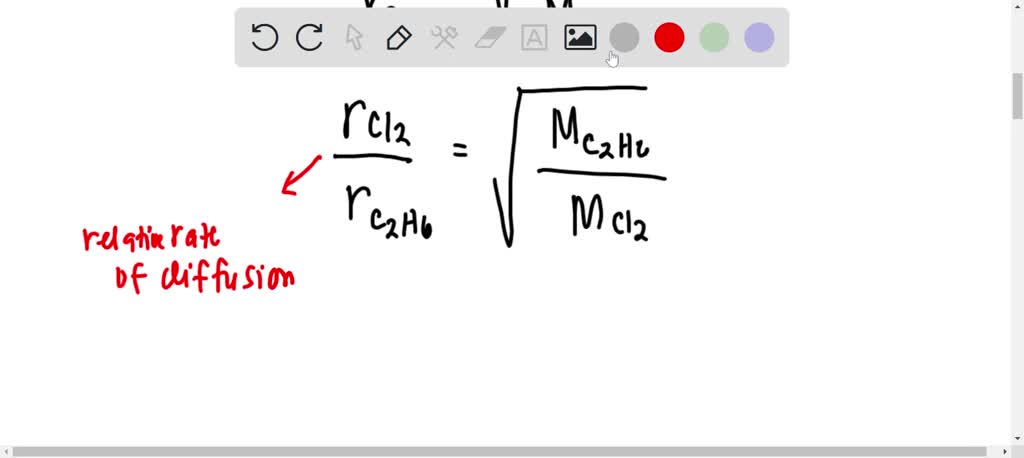
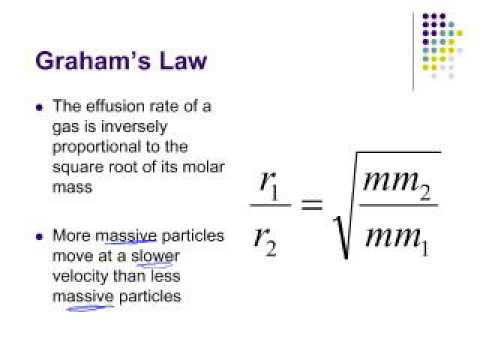
When r, p and M represent rate of diffusion, pressure and molecular mass, respectively, then the ratio of the rates of diffusion (rA/rB) - Sarthaks eConnect | Largest Online Education Community
34. if the ratio of Rates of diffusion of two gases X and Y is 9 : 1 the ratio of their densities is
63.Rate of diffusion of gas X is twice that of gas Y if molecular mass of Y is 64 then the molecular mass of X will be

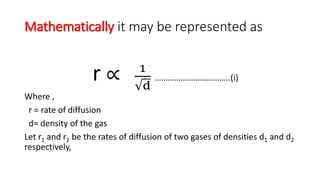
Rate of diffusion of gas is :directly proportional to its molecular massdirectly proportional to square of its molecular massinversely proportional to the square root of its moleculer massdirectly proportional to its vapour

The rates of diffusion of gases A and B of molecular weight 36 and 64 are in the ratioa)9:16b)4:3c)3:4d)16:9Correct answer is option 'B'. Can you explain this answer? - EduRev JEE Question
Rate of diffusion of a saturated hydrocarbon is about 1/6 th of that of hydrogen under similar conditions of temperature and pressure. What is the molecular formula of that hydrocarbon?

Rates of diffusion of two gases A and B are in the ratio 2:1. If the molecular mass of gas A is 16g. Find the...